pH calculation of a buffer solution made from a weak base and its conjugate acid (salt form) - YouTube

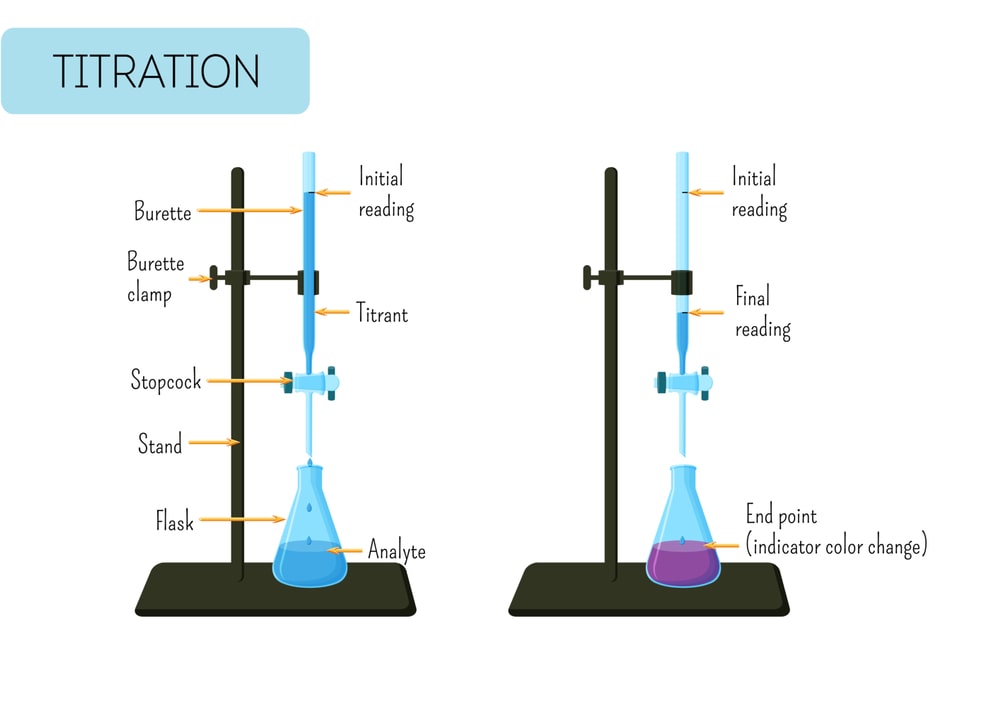
Ammonium-chloride-assay - Assay Of Ammonium Chloride AIM: To determine the percentage purity of - Studocu
CHEM 1332 (A.M. Guloy) CHEMICAL EQUILIBRIA--ACID/BASE Acid/base problems may fall into 4 categories: strong acid/base, weak acid

SOLVED:A buffer contains significant amounts of ammonia and ammonium chloride. Write equations showing how this buffer neutralizes added acid and added base.




![Solved BUFFERS 10. An ammonium[NH3]/ammonium chloride | Chegg.com Solved BUFFERS 10. An ammonium[NH3]/ammonium chloride | Chegg.com](https://media.cheggcdn.com/media/98f/s907x655/98fc93a3-c6f6-4a89-8acb-45d7d9f5eb4d/image.png)