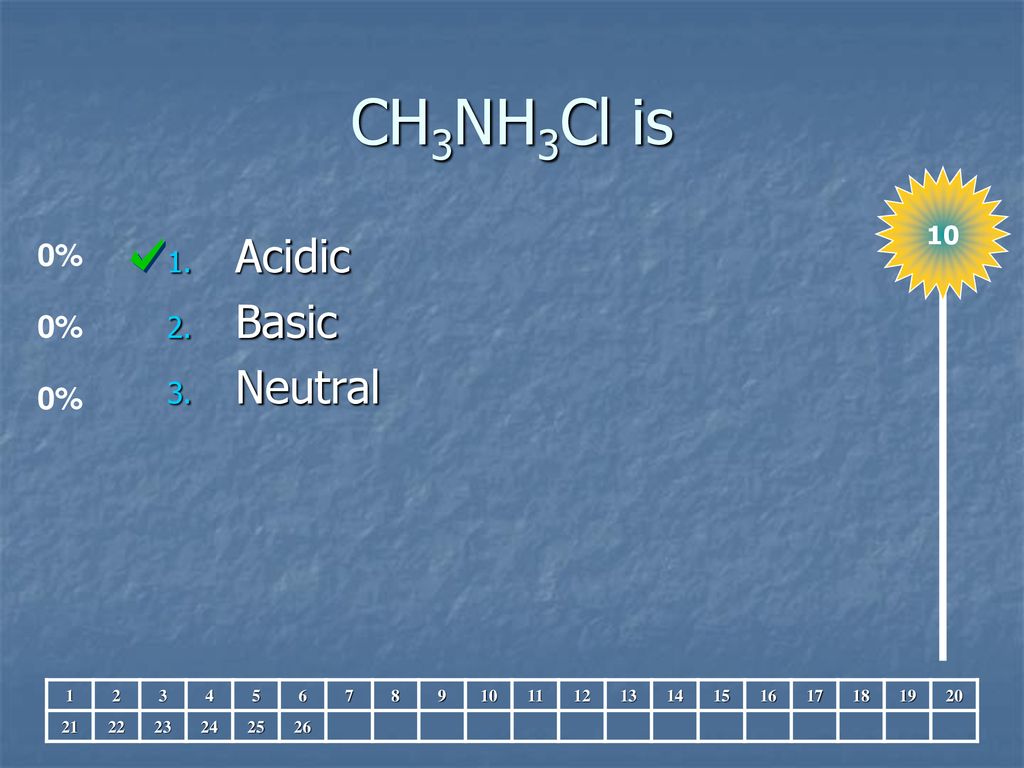
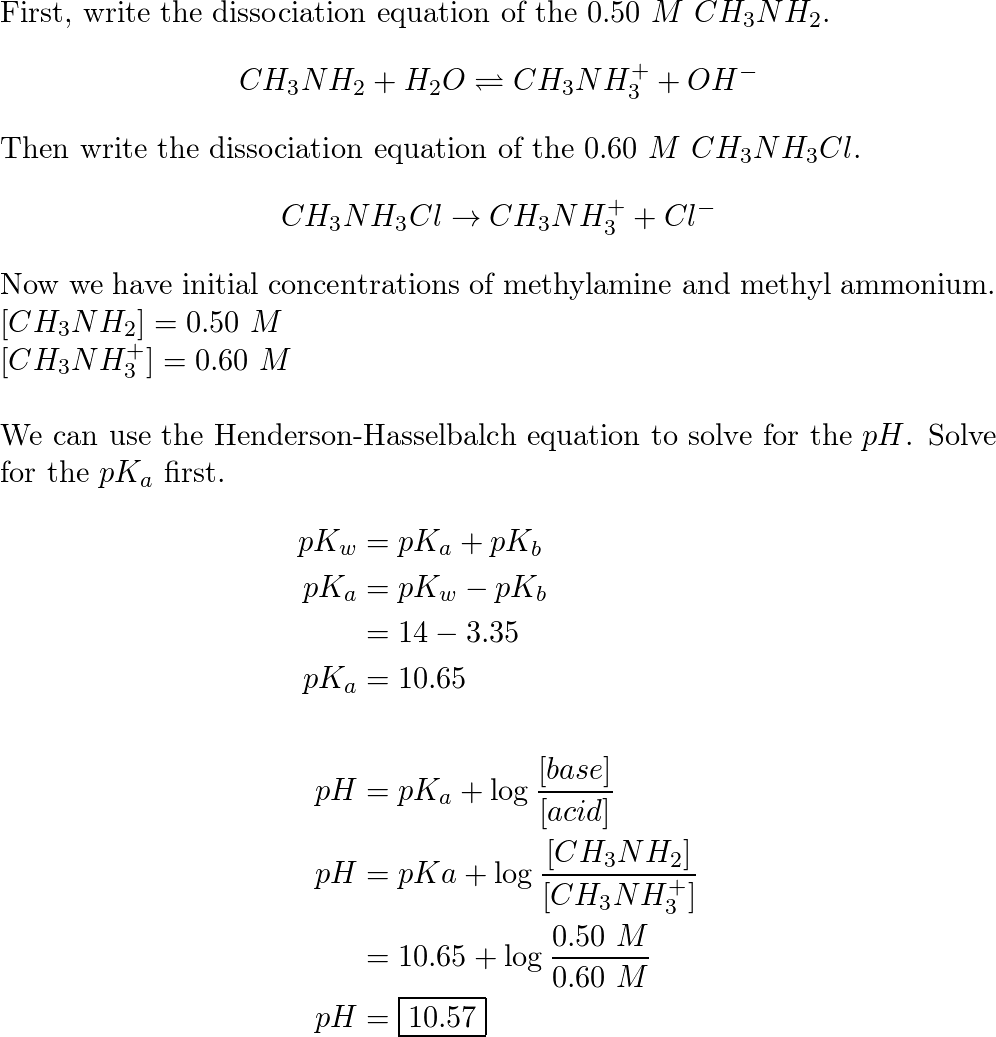Write the net ionic reaction that occurs upon the addition of hno3 to a solution which contains methylamine - Brainly.com

Solved) - Is the solution of KCI acidic, basic or neutral?. Is the solution... (1 Answer) | Transtutors

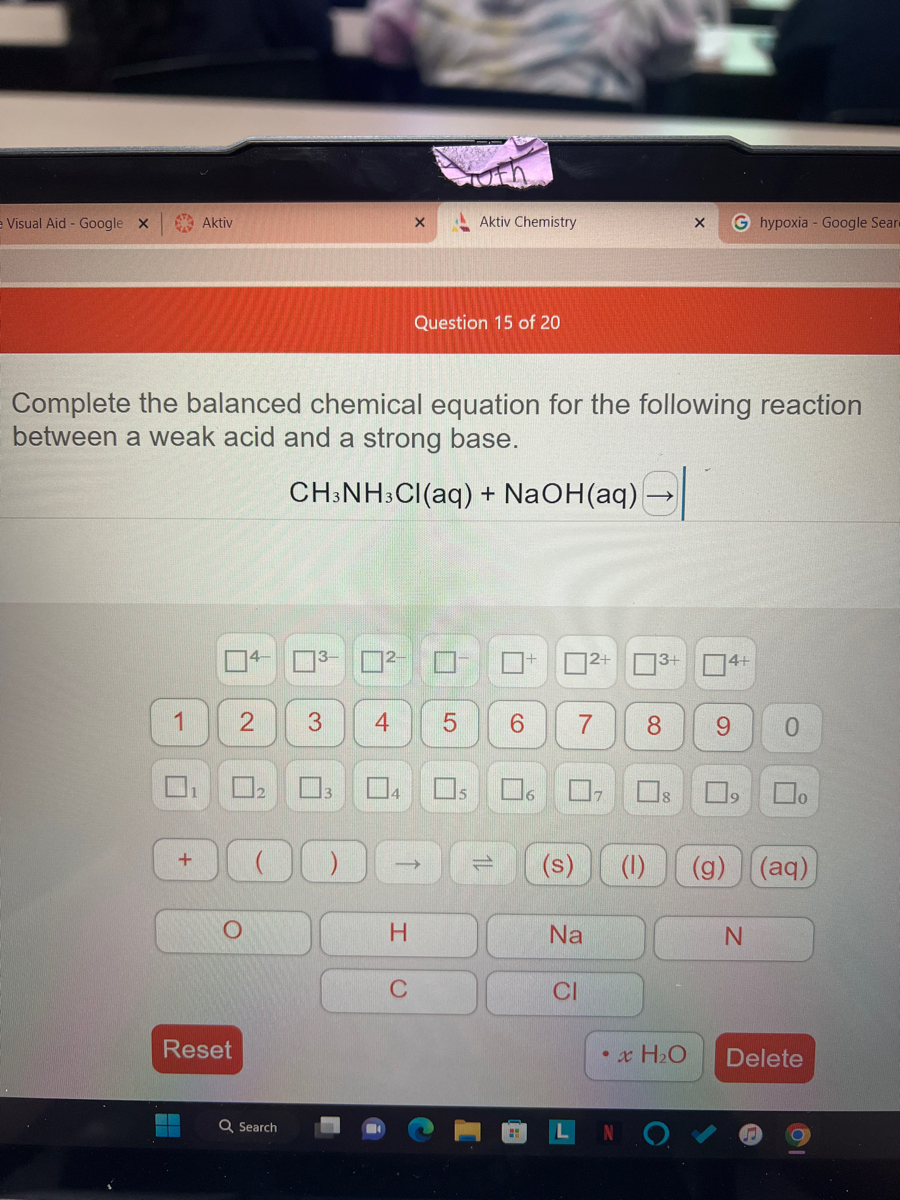
Which of the following mixtures would result in a buffered solution when 1.0 L of each of the two solutions are mixed? Why? : r/chemhelp
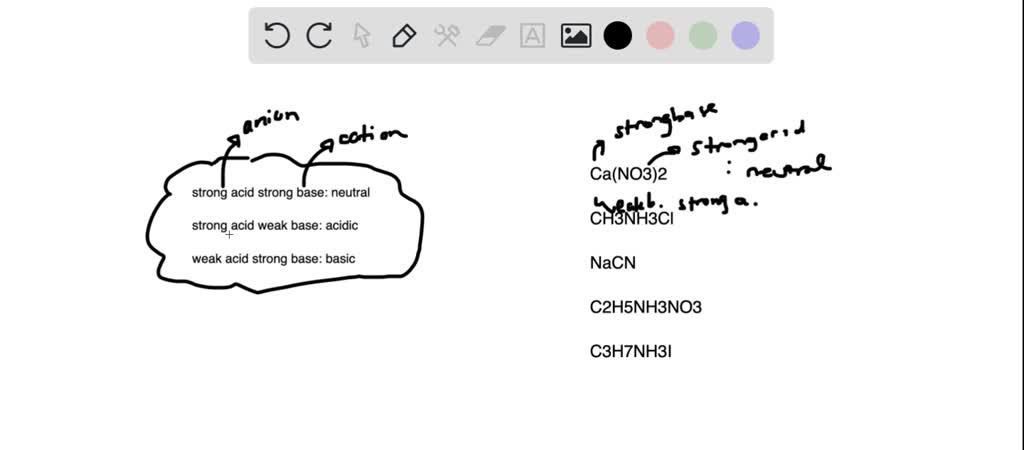
SOLVED: Consider 0.25 M solutions of the following salts. For each salt, indicate whether the solution is acidic, basic, or neutral. Ca(NO3)2 CH3NH3Cl NaCN C2H5NH3NO3 C3H7NH3I
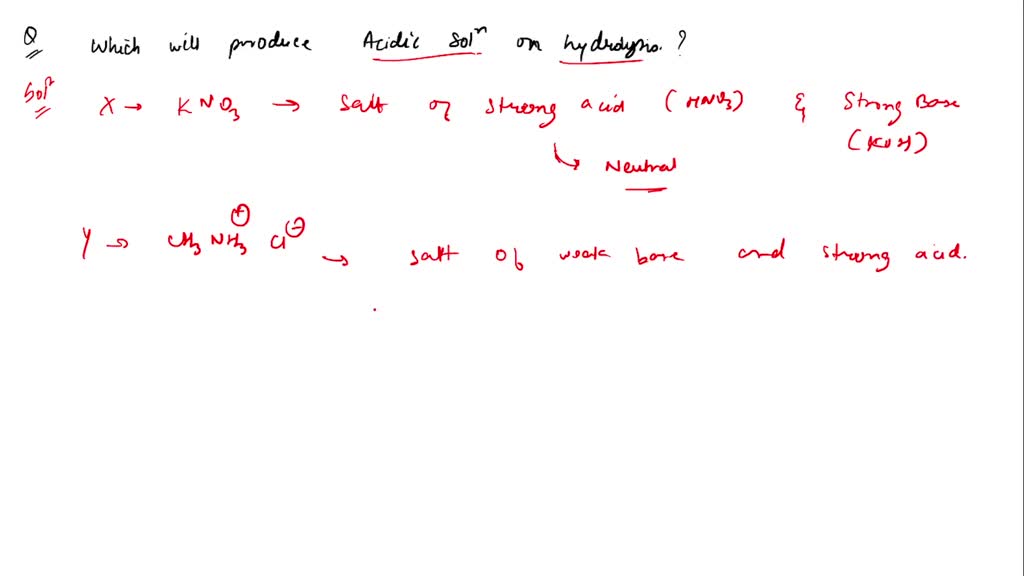
SOLVED: Which of the following compounds, when dissolved in water will produce an acidic solution? X. KNO3 Y. CH3NH3Cl Z. NaHSO4 X only X and Y Y and Z (Correct answer) Z

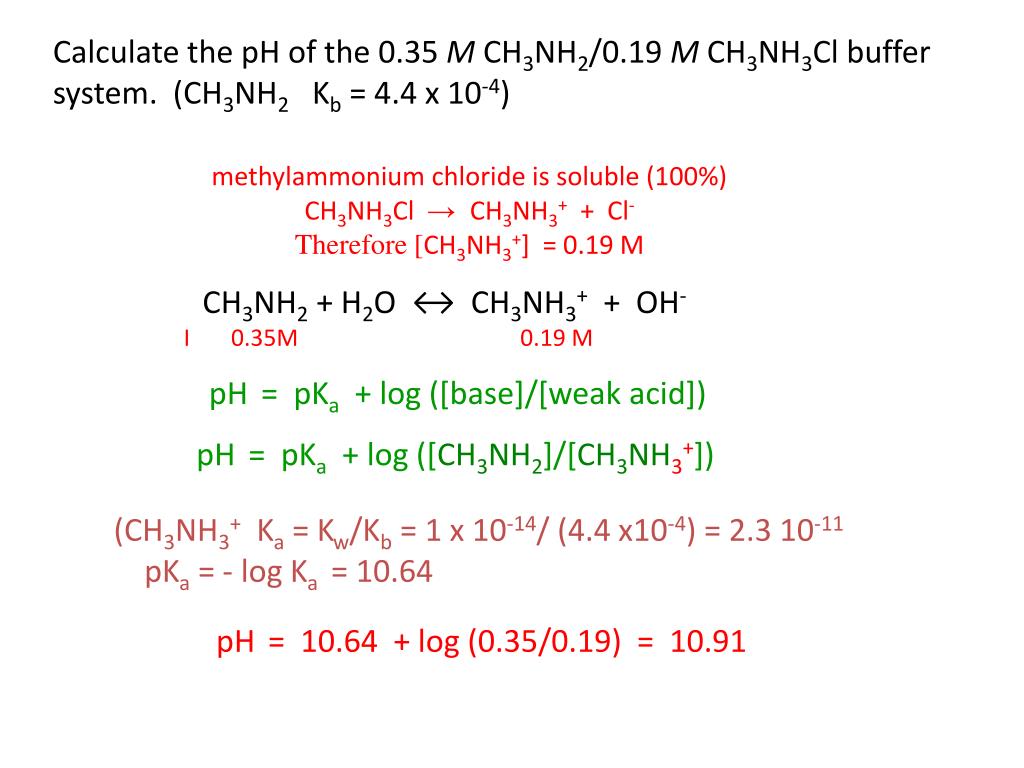
SOLVED: Rank the following salts in order of increasing pH a) NaNO2, CsCl, CH3NH3Cl b) Ca(ClO4), K2S, NH4NO3