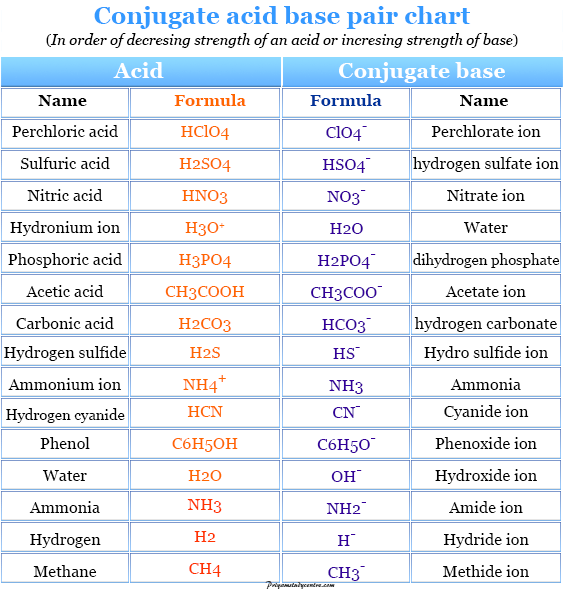
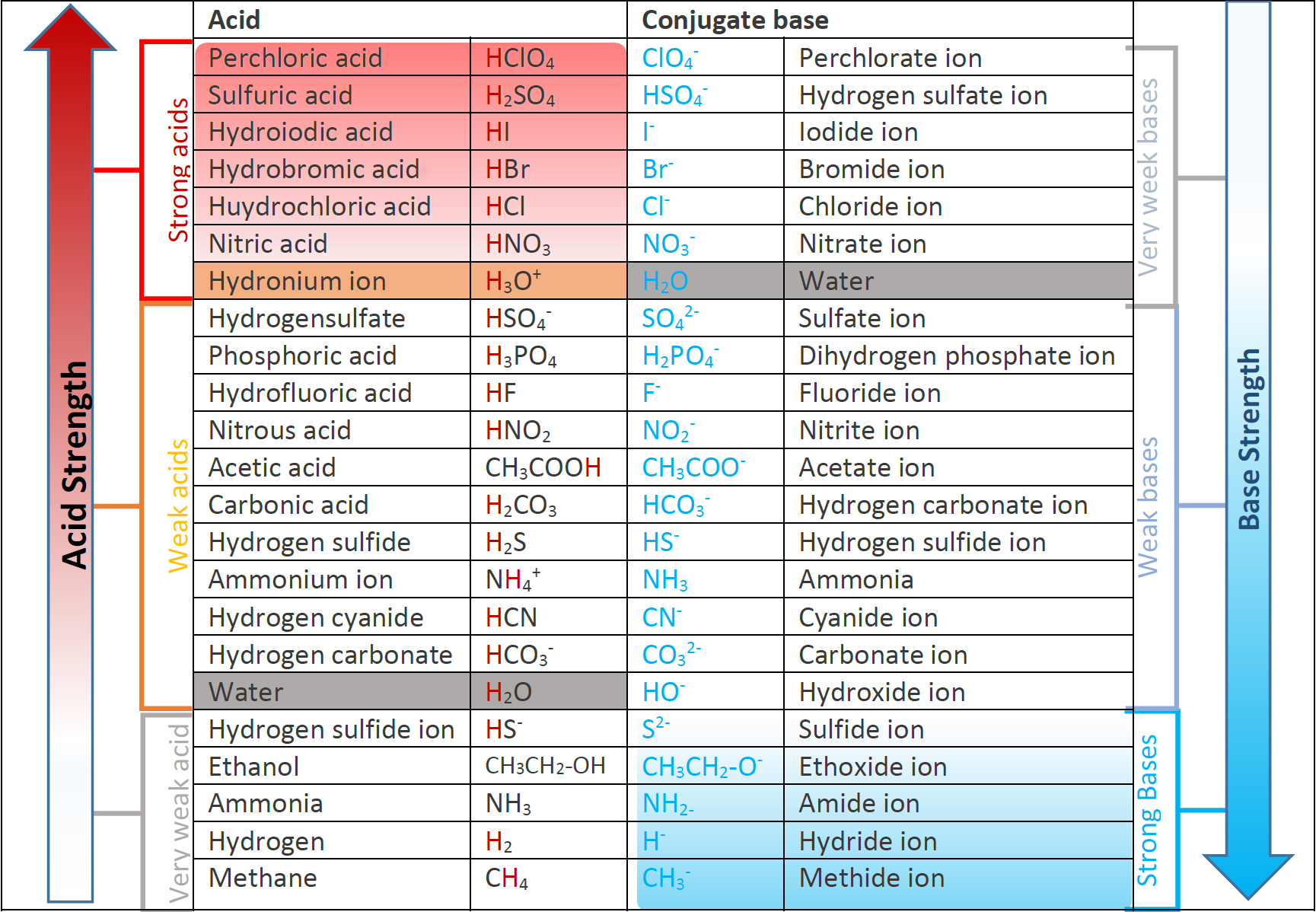
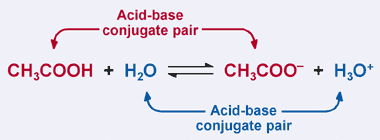
Acid/Base Definitions Arrhenius Model Acids produce hydrogen ions in aqueous solutions Bases produce hydroxide ions in aqueous solutions Bronsted-Lowry. - ppt download
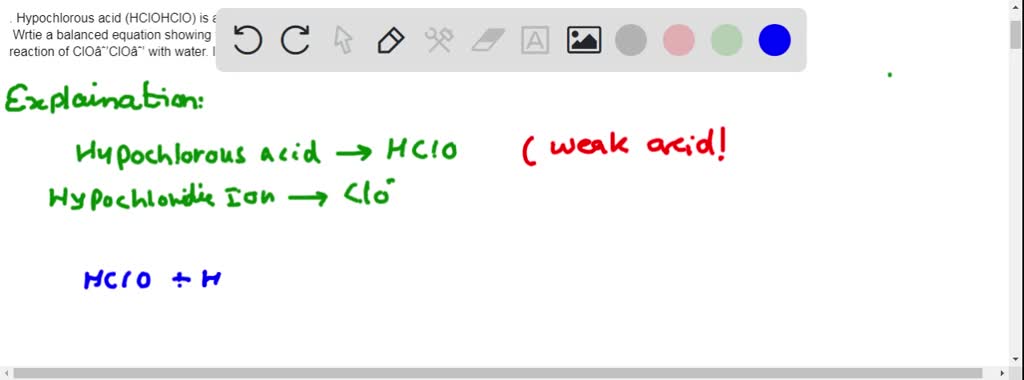
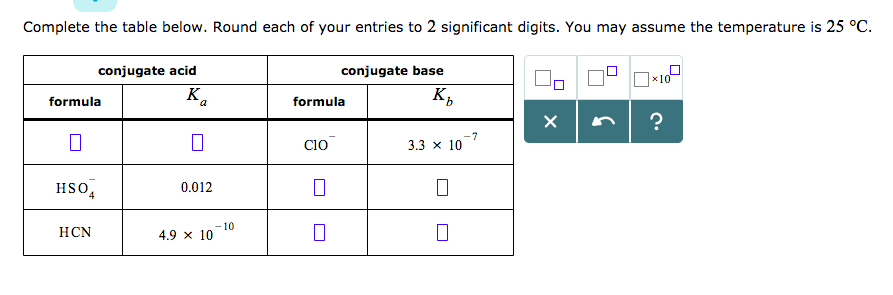
SOLVED: 1. Hypochlorous acid (HClOHClO) is a weak acid. The conjugate base of this acid is the hypochlorite ion (ClO−ClO−). Wrtie a balanced equation showing the reaction of HClOHClO with water. Include

Acids and Bases intro. Acid/Base Definitions Arrhenius Model Acids produce hydrogen ions in aqueous solutions Bases produce hydroxide ions in aqueous. - ppt download