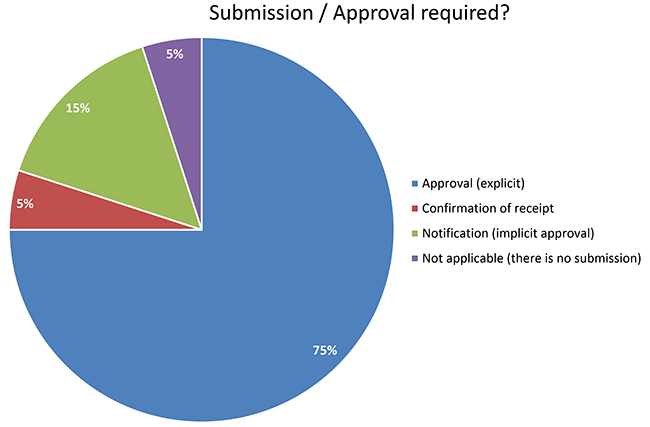
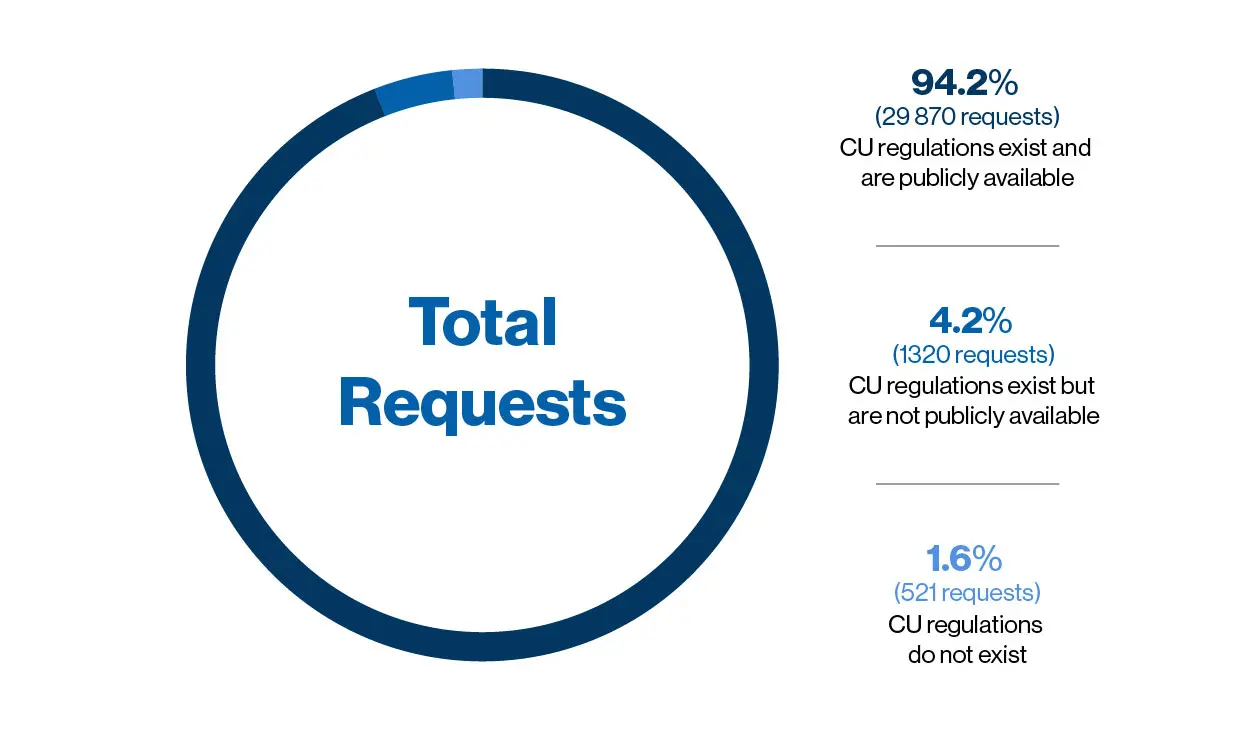
Understanding the challenges and ethical aspects of compassionate use of drugs in emergency situations Goyal PK, Mathur R, Medhi B - Indian J Pharmacol

I'm Willing To Try Anything': Compassionate Use Access To Experimental Drugs And The Misguided Mission Of Right-To-Try Laws | Health Affairs
European Medicines Agency Domenico Scarlattilaan 6 1083 HS Amsterdam The Netherlands Subject: IV Zanamivir Procedure No. EMA/H

Manufacturer's Compassionate Use Policies: Companies with Posted Policies More Than Doubled Since September 2016