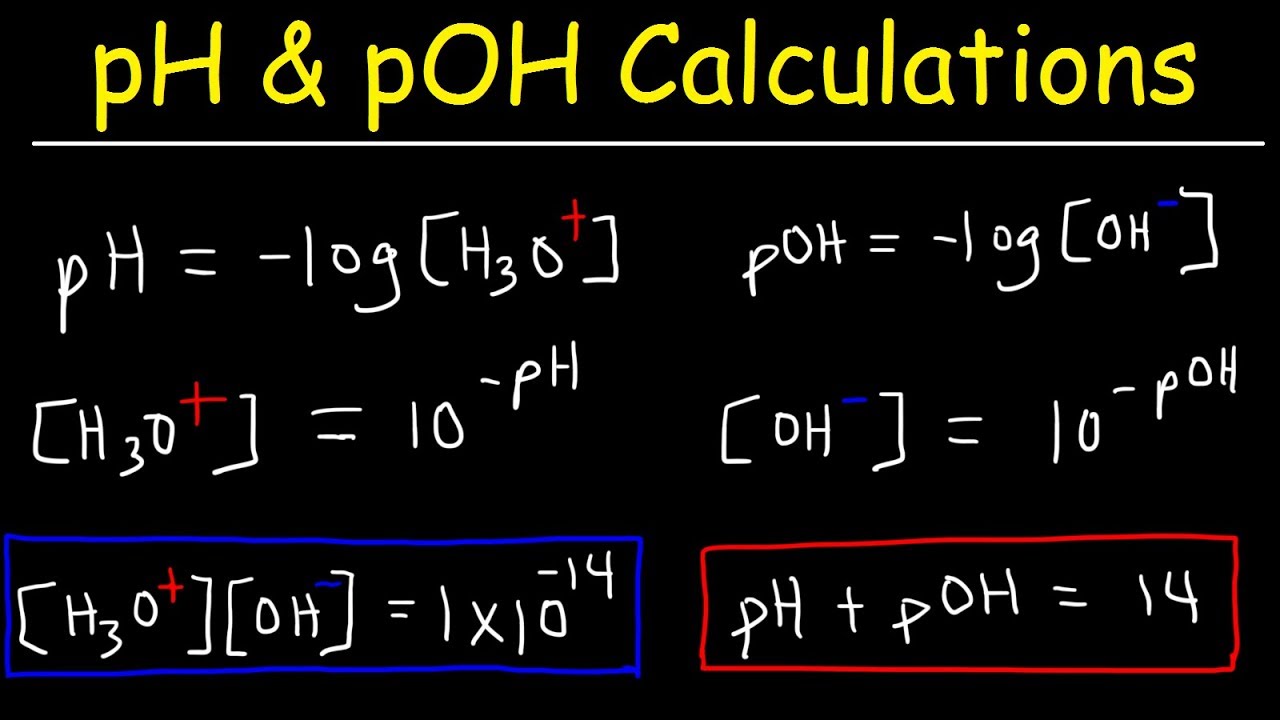
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - YouTube
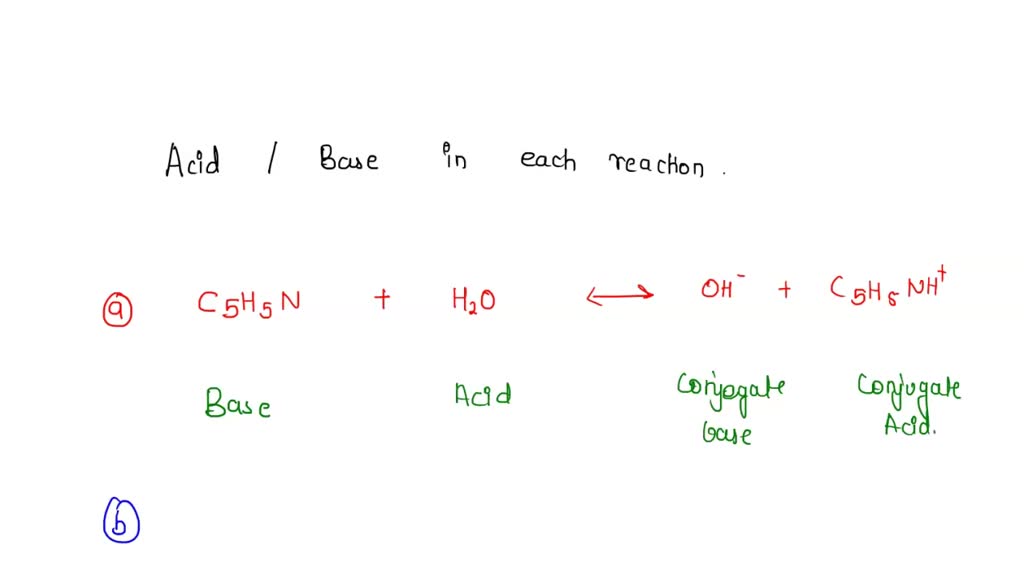
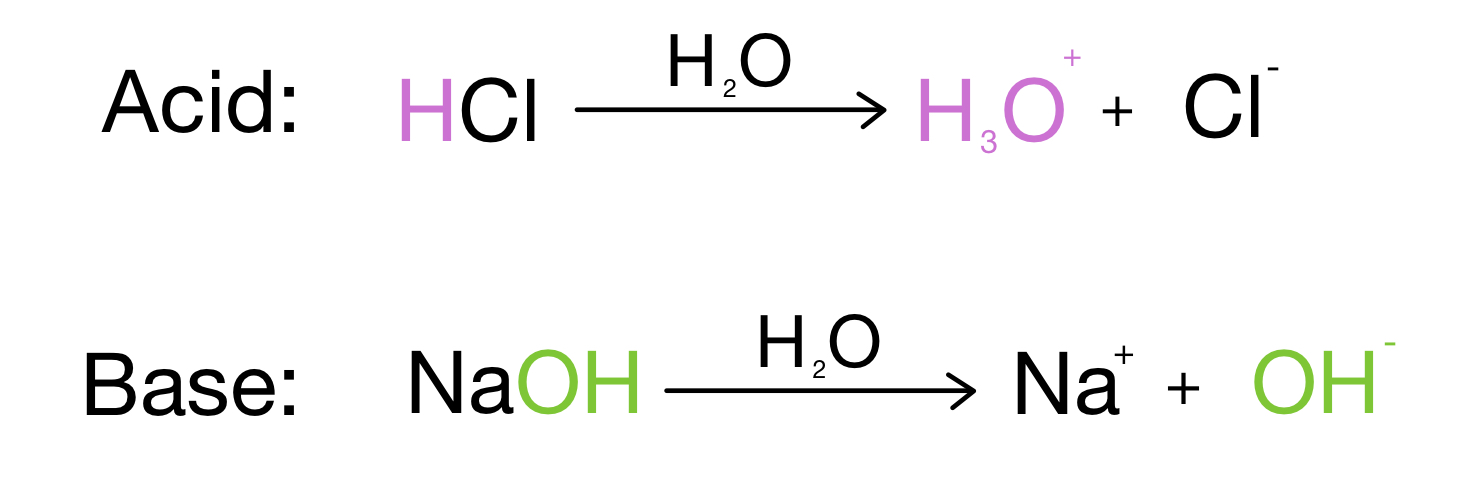
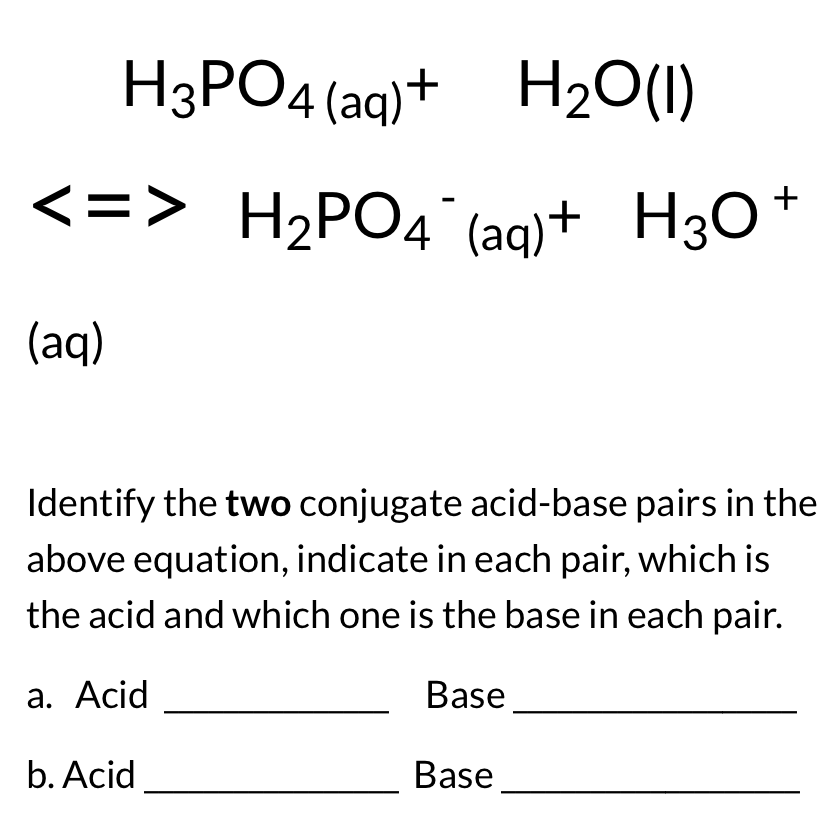
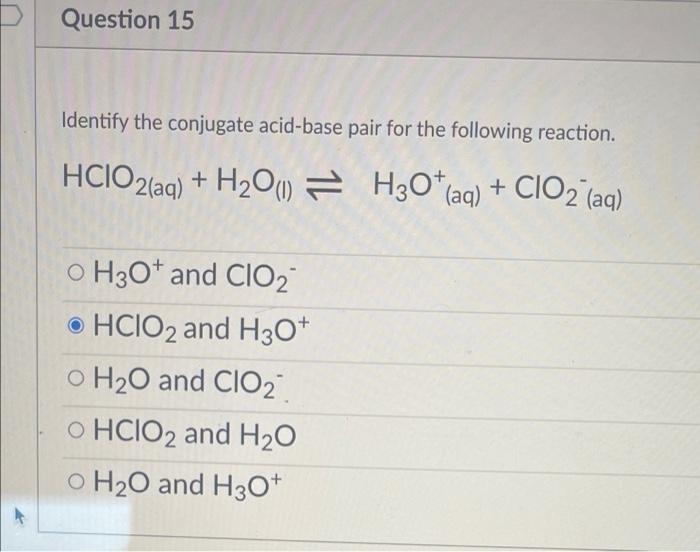
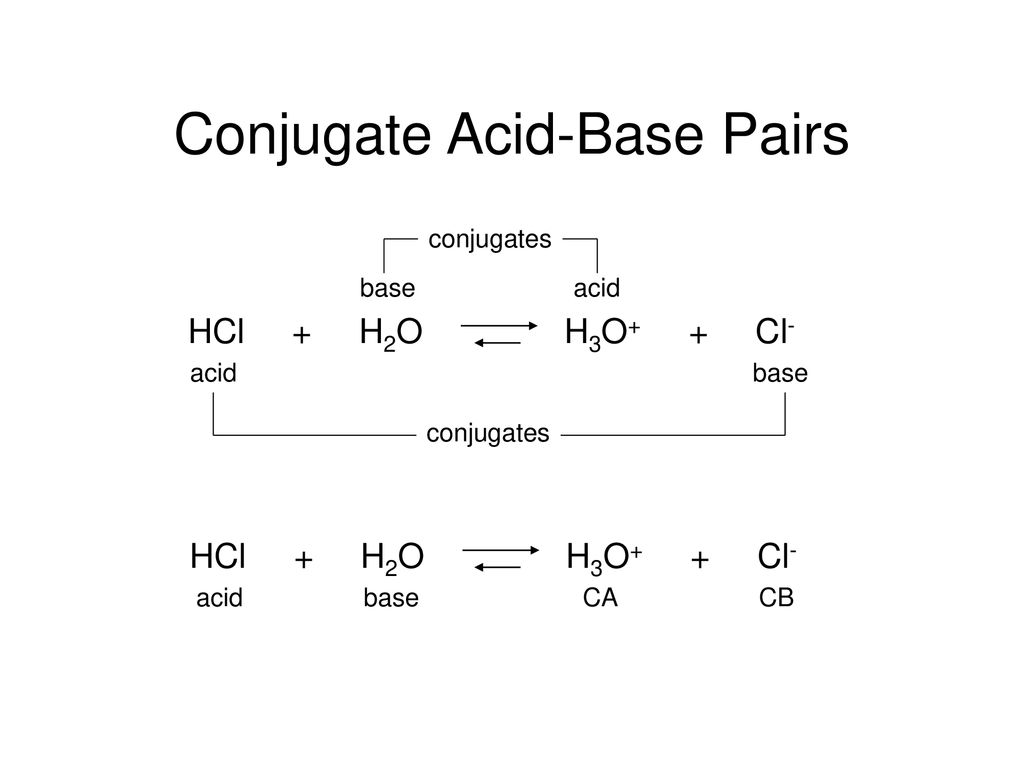
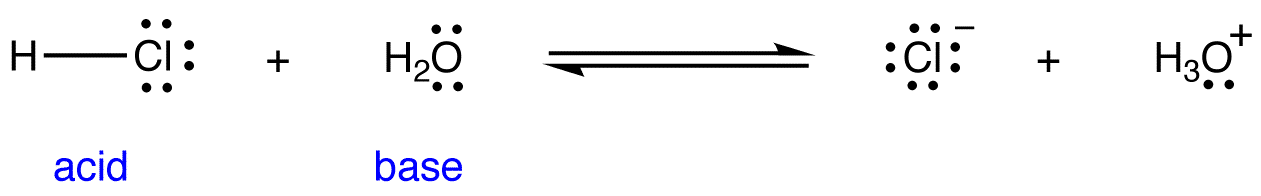
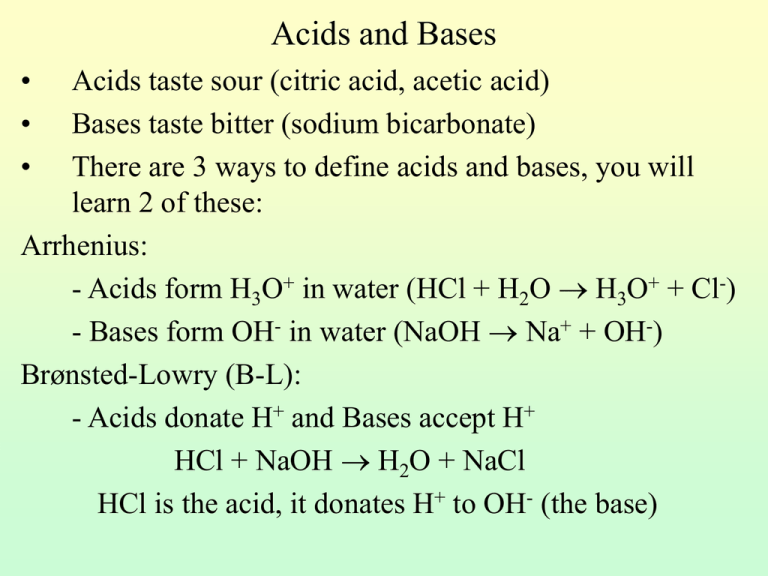
SOLVED: Identify the Brønsted–Lowry acid and base in each reaction: a.C5H5N + H2O <—> OH- + C5H5NH+ b.CH3COOH + H2O <—> H3O+ + C2H3O2- c. KOH + HBr —-> H2O + KBr

.PNG)
![16.6 Finding the [H3O+] & pH of Strong & Weak Acid Solutions - YouTube 16.6 Finding the [H3O+] & pH of Strong & Weak Acid Solutions - YouTube](https://i.ytimg.com/vi/UyBvt7-PDi0/hqdefault.jpg)
![Calculating pH, pOH, [H+], [H3O+], [OH-] of Acids and Bases - Practice - YouTube Calculating pH, pOH, [H+], [H3O+], [OH-] of Acids and Bases - Practice - YouTube](https://i.ytimg.com/vi/UiK37I159fc/maxresdefault.jpg)
![16.6: Finding the [H3O+] and pH of Strong and Weak Acid Solutions - Chemistry LibreTexts 16.6: Finding the [H3O+] and pH of Strong and Weak Acid Solutions - Chemistry LibreTexts](https://i.ytimg.com/vi/y7DTjgrcP-0/maxresdefault.jpg)
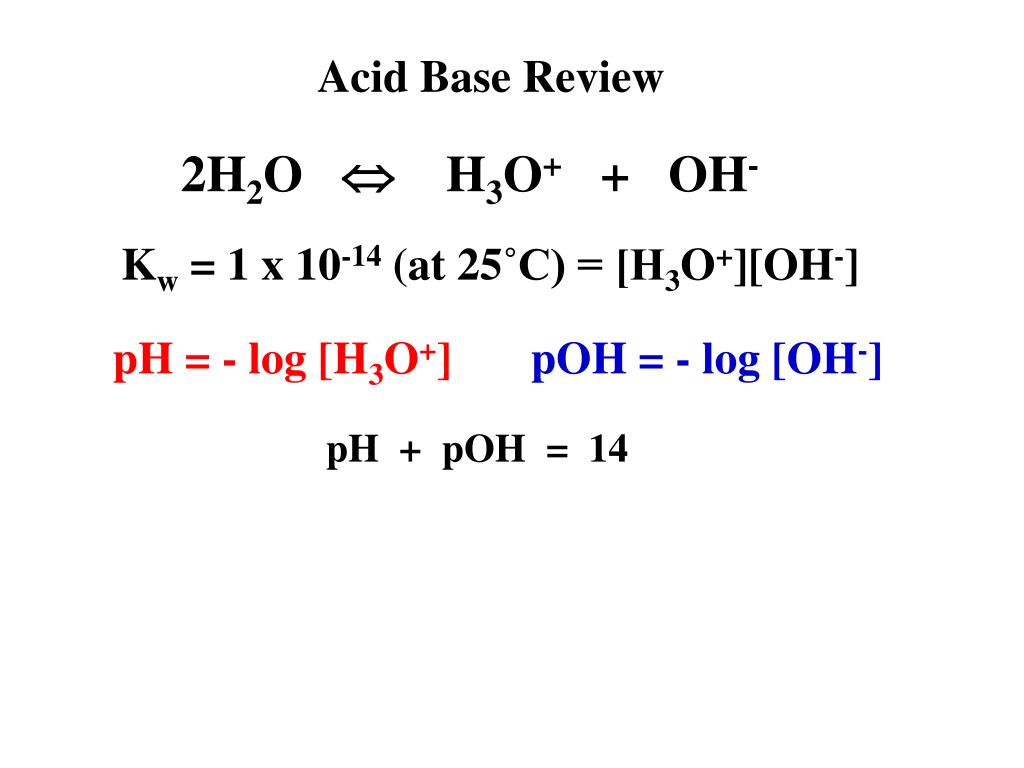
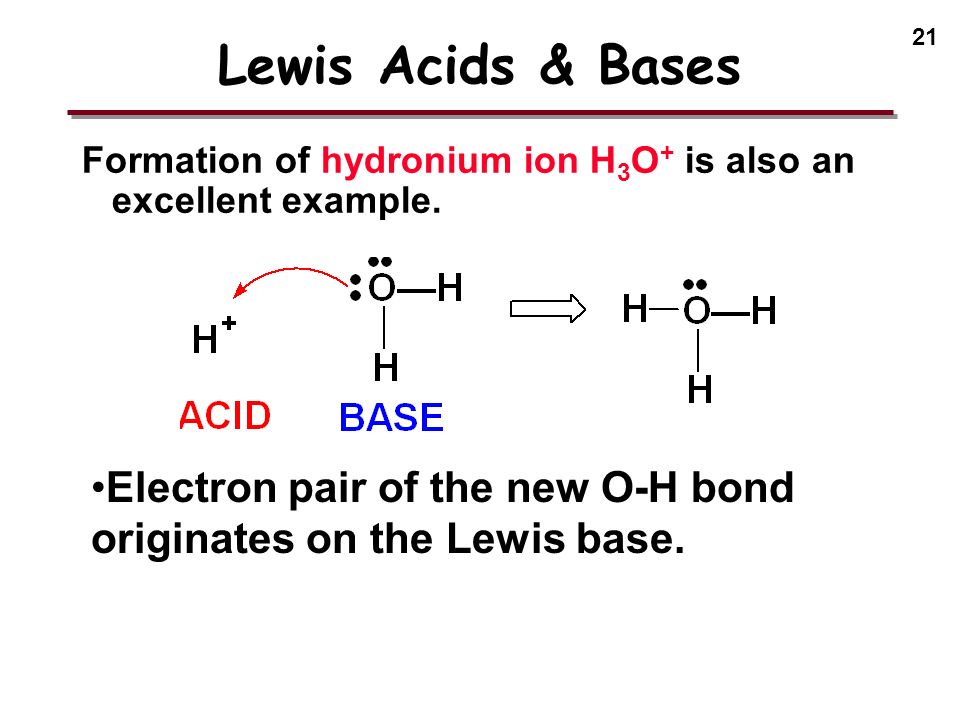
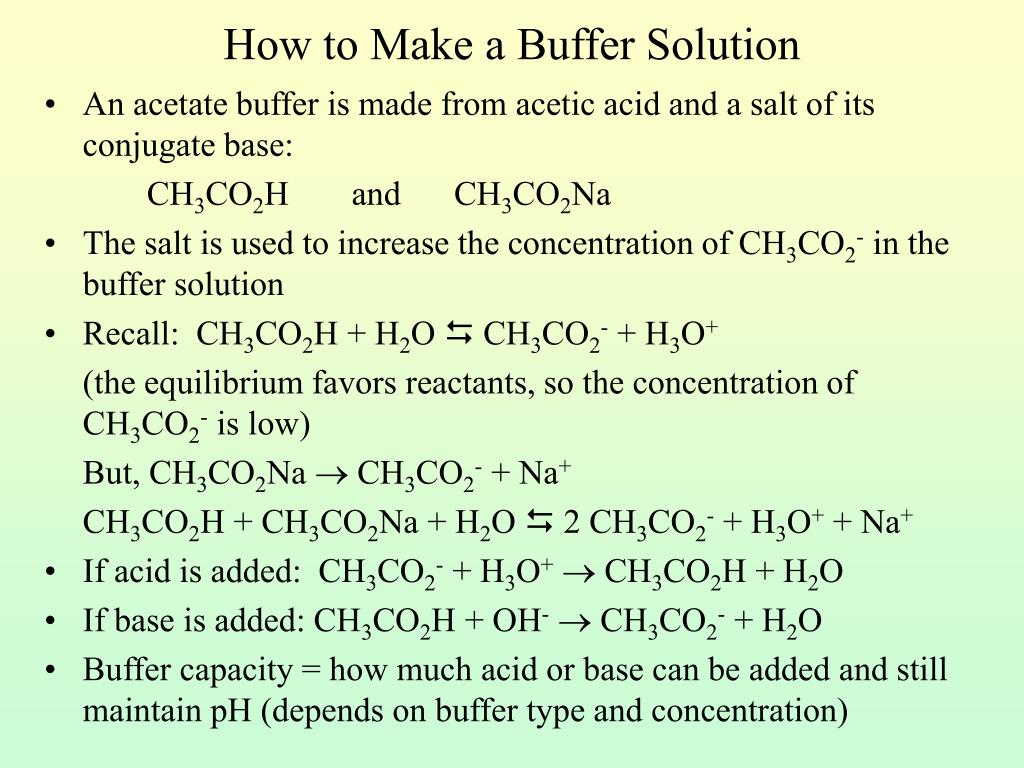




![Solved [H3O+] [OH-] pH pОН Acidic, Base or Neutral? | Chegg.com Solved [H3O+] [OH-] pH pОН Acidic, Base or Neutral? | Chegg.com](https://media.cheggcdn.com/media/f34/f34e841e-96b5-4eae-8369-3ddf8b4861c0/phpT8rw9L)